In my early article Agricultural borates primer: Necessary for proper plant nutrition, I discussed the different types of borates, solubility by type, and the types of borax U.S. Borax produces. In this post, I’ll cover how borate solubility affects plant absorption and recent research in the field.
As we know, boron is an essential micronutrient for crop production and plays a key role in a diverse range of plant functions including cell wall formation, permeability and stability, membrane function and cell division, development of flowers and fruits, and growth of roots and new tissues in plants.
Relative susceptibility of crops to boron
In general, dicotyledonous plants have a greater demand for boron and also have a greater capacity to absorb this nutrient from the soil, when compared to monocotyledonous plants.
Boron deficiency can significantly affect crop yields. This is especially true in tropical regions where most soils are acidic and have low or medium boron levels.
Borate solubility by type
To refresh your memory, the more soluble a product is the more boron is available to the plant. The solubility of borates depends on the source material and the interaction of the boron with sodium (Na), calcium (Ca), and magnesium (Mg). The more Mg and Ca a borate product has the less soluble this mineral will be.
Product/fertilizer
|
Chemical formula
|
Solubility in water
(g/L at 20° C)
|
| Borates |
| Hydroboracite |
CaO·MgO·3B2O3 ·6H2O |
0.8 g/L |
| Colemanite |
2CaO·3B2O3 ·5H2O |
4.7 g/L |
| Ulexite |
Na2O·2CaO·5B2O3 ·16H2O |
10.9 g/L |
| Sodium Tetraborate Pentahydrate (Granubor®) |
Na2B4O7·5H2O |
26.5 g/L |
| Boric Acid (Optibor® TG) |
H3BO3 |
47.2 g/L |
| Disodium Octaborate Tetraborate (Solubor®) |
Na2B8O13·4H2O |
97 g/L |
| NPK fertilizers |
| Muriate of Potash |
KCl |
344 g/L |
| Urea |
CH4N2O |
1080 g/L |
What form of boron do plants absorb from soil?
All refined (sodium borates) and unrefined borates (ulexite and colemanite) will be partially or completely converted into boric acid (H3BO3) in the soil. So, regardless of the boron source applied to the soil, plants will always absorb boron in this form.
When farmers apply ulexite to the soil, part of this mineral will be converted into boric acid, which will be absorbed by the plants. But other molecules of boron remain linked to the Ca molecule forming a compound which is not bioavailable for plant uptake. Because refined borates don’t have Ca in their composition, when farmers apply sodium tetraborate pentahydrate (Granubor) to the soil, 100% of available boron will be converted to boric acid within 40 weeks (280 days).
Does boron type matter? Research proves it does
In order to help clarify doubts about different borates on the market, Barth & Haliski (2020) conducted a percolation study to compare the rate of boron release from various sources. The field trial was conducted at the ABC Foundation laboratory in Castro/PR, Brazil.
The soil used was 52.5% sand, 35.3% clay, and 12.2% silt. The experiment was conducted under two conditions:
- Without limestone soil correction (pH 4.8)
- With limestone correction (pH 5.7)
Sources tested included:
- U.S. Borax’s refined borate fertilizer Granubor (15% B): A water-soluble fertilizer created from sodium tetraborate pentahydrate
- Colemanite (calcium borate): A mineral with low solubility in water
- A technology based on potassium chloride (KCl) + two sources of boron in the same granule (58% K2O and 0.5% B): The two sources of boron present in the compacted KCl+B product are made from on anhydrous sodium tetraborate (50%) and colemanite (50%)
The results show that colemanite released only 6.9% of the boron after 140 days in soil at pH 4.8 and 6.4% in soil at pH 5.7 (Figures 1 and 2). The compacted KCl+B product released 57.5% and 60.1% of boron after 140 days in soil with pH 4.8 and 5.7, respectively; the boron release was not complete because this fertilizer has 50% calcium borate (colemanite) in its composition, which solubility in water is extremely low (Figures 1 and 2). Granubor released 95.5% and 92.9% after 140 days, respectively, (Figures 1 and 2). These results show that the boron release rate from Granubor is better related to the boron uptake by annual crops such as cotton, soybean, and corn, and coincides with the reproductive period of these crops.
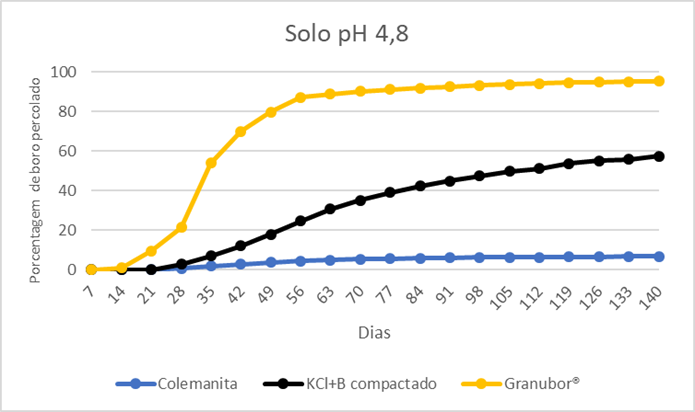
Figure 1. Percentage of boron percolated in a soil under pH 4.8. Source: ABC Foundation, 2020.
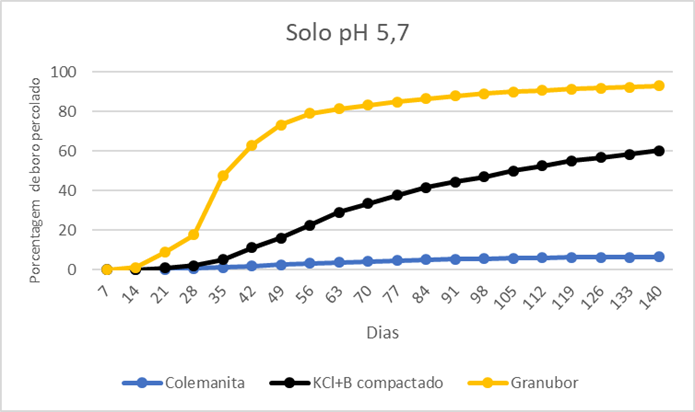
Figure 2. Percentage of boron percolated in a soil under pH 5.7. Source: ABC Foundation, 2020.
In earlier research, Barth & Suyama (2017) conducted a percolation study to compare the rate of boron release from various boron sources. The work was conducted at Fundação ABC laboratory in Castro/PR, Brazil. The experiment was conducted under two conditions:
- In sandy soil (pH 4.7)
- In clayey soil (pH 4.1)
The boron sources tested were:
- U.S. Borax’s refined borate fertilizer Granubor (15% B): A water-soluble fertilizer created from sodium tetraborate pentahydrate
- Ulexite: Granulated 10% B originating from Argentina
- Another source of granulated ulexite 10% B originating in Bolivia: Sodium-calcium borate which is only partially water soluble
The results showed that ulexite from Argentina released 45.4% of boron after 280 days (40 weeks) in the sandy soil (Figure 1) and 35.2% in the clayey soil (Figure 2). Bolivian ulexite released 39.8% of boron after 280 days in sandy soil (Figure 1) and 34.4% in clayey soil (Figure 2). Granubor fertilizer released 99.7% B in the sandy soil (Figure 1) and 99.5% boron in the clayey soil (Figure 2), after 280 days. On average, ulexites released 38.7% of boron after 280 days, while Granubor released 99.6%.
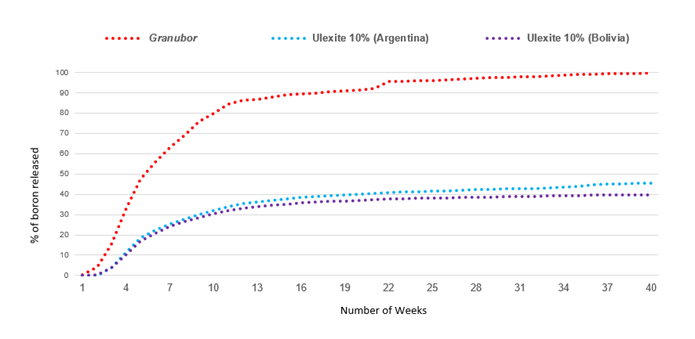 Figure 1. Percentage of boron percolated in a sandy soil with pH 4.7.
Figure 1. Percentage of boron percolated in a sandy soil with pH 4.7.
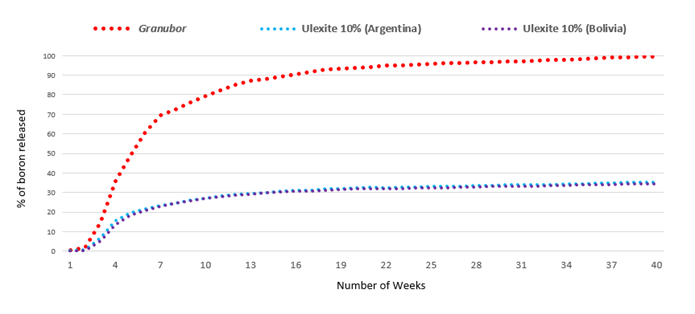 Figure 2. Percentage of boron percolated in a clayey soil with pH 4.1.
Figure 2. Percentage of boron percolated in a clayey soil with pH 4.1.
Dissolution rates for different types of borax
Although borax decahydrate and borax pentahydrate, come from the same ore, they are different products. U.S. Borax produces three different forms of “borax:”
- Borax decahydrate (Na2B4O7 • 10H2O): About 11.4% boron
- Borax pentahydrate (Na2B4O7 • 5H2O): About 15% boron
- Anhydrous borax (Na2B4O7): About 21.3% boron
Time for 100% dissolution for different types of “borax” at 20° C
| Product |
Chemical formulation |
Time for dissolution |
| Borax decahydrate |
Na2B4O7 • 10H2O |
8 min 30s |
| Borax pentahydrate (Granubor) |
Na2B4O7 • 5H2O |
80 min 30s |
| Anhydrous borax (Anybor®) |
Na2B4O7 |
23 hours |
The water solubility of each type of borax will depend upon the degree of hydration. Because borax decahydrate has a greater solubility in water and a lower percentage of boron, it is not widely used in agriculture. Rather it is mainly used in a variety of industrial manufacturing end uses, including as a laundry detergent booster.
On the other hand, borax pentahydrate (sold commercially by U.S. Borax as Granubor and Fertibor®) and anhydrous borax fine (Anhybor) are very efficient sources for use as fertilizers.
Resources