Authors:
- Fabiano Silvestrin, Principal Advisor, Global Market Development Agriculture
- Eduardo Saldanha, Agriculture Development Specialist, Latin America
Arsenic (As) is a naturally occurring, toxic heavy metal dispersed in the environment through a variety of industrial, mining, and agricultural activities.
In the natural environment, arsenic pollutes the soil and contaminates water, thus posing a serious threat to flora and fauna, including plants, animals, and humans. Arsenic groundwater contamination is a serious problem in many regions of the world, where millions of humans are at the risk of arsenic-poisoning due to drinking contaminated water. Arsenic also severely affects the growth and development of plants.
Heavy metals, in general, cause toxicity and cellular disruption in plant species through induction of oxidative stress via increased generation of ROS, such as 1 O2 (singlet oxygen), O2 (superoxide radical), OH (hydroxyl radical), and H2O2. Arsenic induces oxidative stress resulting in cellular damage in terms of enhanced lipid peroxidation and H2O2 accumulation.
Agricultural research into arsenic
A study by Singh et al. 2007, evaluating two concentrations of arsenic in bean plants, showed strong effects in reducing root growth (Table 1). An exposure to arsenic caused a significant reduction in root band shoot lengths of mung bean seedlings (Table 1). This inhibitory effect was more pronounced on root length than on shoot length. The root length decreased by more than 63% and 82% in response to 10.0 and 50.0 lM arsenic, respectively.
Table 1. Effect of arsenic (As) on seedling growth in mung bean
| Arsenic |
Root length |
Shoot length |
Root/shoot |
| µM |
cm 1 |
cm |
ratio |
| 0.0 |
3.37 a (0)2 |
4.40 (0) |
0.77 a |
| 10.0 |
1.23 b (63.5) |
2.23 (49.3) |
0.55 b |
| 50.0 |
0.6 c (82.2) |
1.18 (73.2) |
0.55 c |
1 The data were recorded after 7 days’ exposure to As; means with common letters are not significantly different at P _ 0.05, according to Tukey’s test.
2 Figures in parenthesis represent the percent decrease over the control.
The reduction in root elongation was accompanied by anatomical changes in mung bean roots, the results showed that arsenic also induced anatomical changes in mung bean roots (Figure 1). In comparison to untreated control roots where all the epidermal cells were intact and the root hairs were turgid, arsenic exposure resulted in a severe decrease (at 10.0 lM) in, or a complete absence (at 50.0 lM) of root hairs. Arsenic caused damage to the epidermal cells and the cortex, with these cells losing their shape and showing signs of shriveling and disintegration.
Figure 1
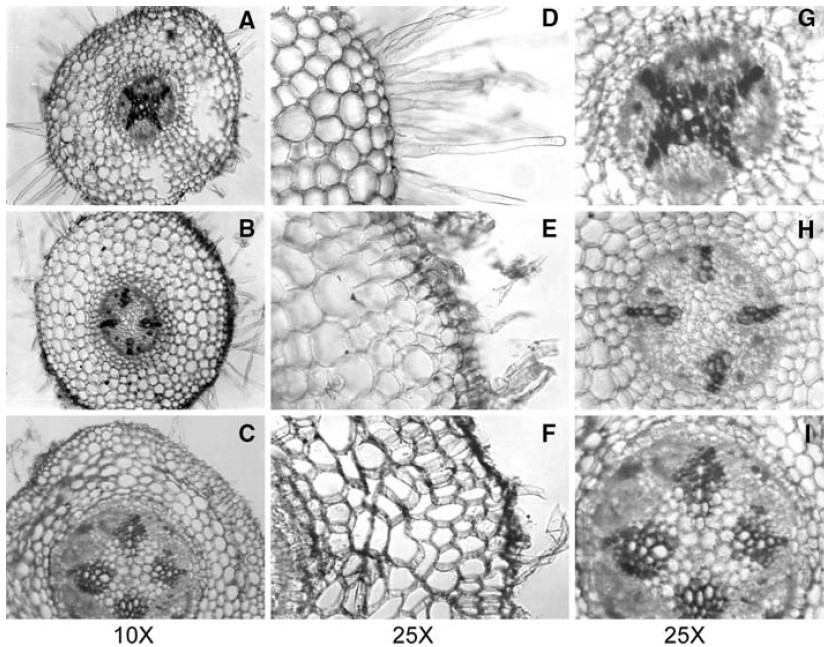
Figure 1: Cross-sections of mung bean roots showing anatomical changes upon exposure to 0.0 (A, D, G), 10.0 (B, E, H) and 50.0 µM (C, F, I) arsenic. First column, cross-section of the root; middle column, close-up of the epidermal region showing epiblema, root hairs and cortical cells; last column, close-up of the central stellar portion. Source: Singh HP, et al. 2007.
Other studies have shown that arsenic can accumulate in the soil and water, leading to higher levels in plants and causing toxicity shown below.
Figure 2
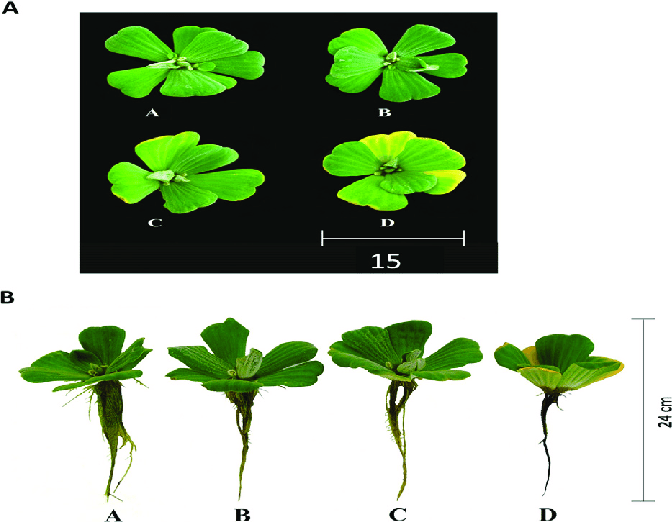
Figure 2: Pistia stratiotes exposed to three arsenic concentrations after four days. A (control), B (5 µM of As), C (10 µM of As), and D (20 µM of As) 1. Visual symptoms of arsenic toxicity in leaves, 2. Visual symptoms of arsenic toxicity in root. Source: Campos, et al. 2019.
Damage to the plant’s growth when exposed to arsenic:
Figure 3

Figure 3: Effect of arsenic in the nutrient solution on Cajanus cajan roots. Treatment A (control), B (with 1.5 mg L-1 of As). Treatment A (normal root and several lateral root branches), B (brownish roots with unusual short lateral branch roots). Source: Pita et al. 2015.
Figure 4
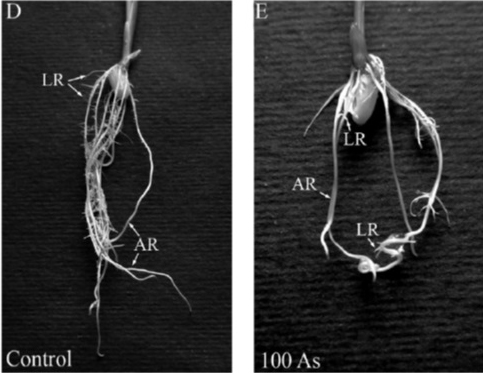
Figure 4: Effect of arsenic on Oryza sativa roots. Control (untreated), 00 As (treated with 100 μM of arsenic). Source: Ronzan et al. 2018.
Arsenic and boron fertilizers
Arsenic levels vary from region to region. And, it should come as no surprise that different borate sources have different levels of arsenic. Arsenic content in borates can also vary within the same mine.
The use of unrefined sources of B in agriculture
When farmers apply unrefined boron (ulexite and/or colemanite) to their crops, impurities such as arsenic are present in the product. Like fertilizer materials, the arsenic is taken up from the soil by the plant.
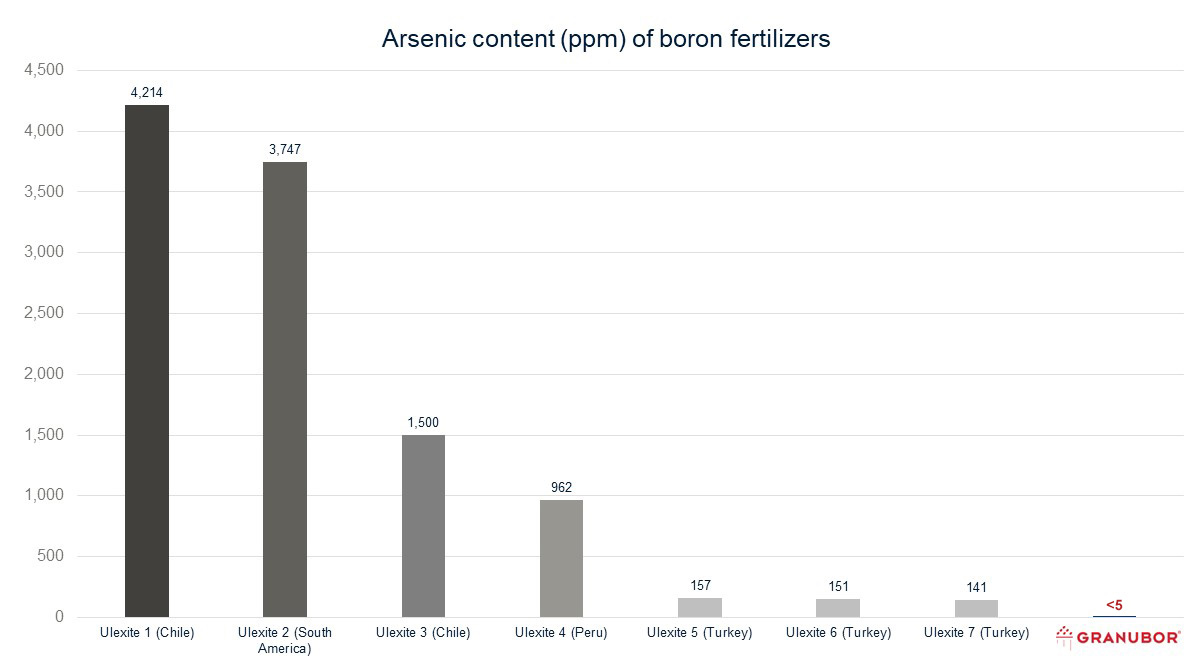
Source: Sample testing performed at the U.S. Borax Quality Lab in Boron, CA and Denver, CO, USA, Fundacão ABC Lab in Castro, PR, Brazil
Refined borates are different. Because the refining process removes arsenic and other heavy metals from the original ore, the final refined fertilizer product is pure and safer for agriculture use. Learn more about the value of refined borates.
Refined borates from U.S. Borax
U.S. Borax products are fully refined to remove impurities and maximize potential plant uptake. This process results in a pure, natural product that provides optimized nutrition throughout the growing season.
The U.S. Borax refining process involves several important steps:
- We mine high-quality sodium-borate ore that is naturally low in calcium and other contaminants
- We dissolve the ore in water
- We settle the dissolved borate liquor to remove impurities
- We cool the liquor and then further wash and filter the resulting crystal slurry
- We dry the refined slurry to produce dry borate crystals
- We check our products for purity, solubility, consistency, and optimal granulation
- We constantly conduct and review field tests and other research to improve our products and services
If you buy unrefined borates from other companies, make sure you send samples of your boron fertilizers to a lab for arsenic analysis to ensure you don’t add additional heavy metals to your field.
U.S. Borax borates and OMRI certificates
For organic growers who require refined boron, U.S. Borax fertilizers are listed appropriate by the Organic Materials Review Institute (OMRI®). This international, nonprofit organization determines which input products are acceptable for use on certified organic crops under the USDA National Organic Program, the Canadian Organic Standards, and the Mexico Organic Products Law. Learn more about U.S. Borax OMRI Listed products.
Contact your U.S. Borax regional sales manager for more information about the refined borate fertilizers we offer.
Resources
References
- Campos FV, Oliveira JA, Silva AA, Ribeiro C, dos Santos FF. 2019. Phytoremediation of arsenite-contaminated environments: is Pistia stratiotes L. a useful tool? Ecological Indicators. 104:794-801.
- Li WX, Chen TB, Huang ZC, Lei M, Liao XY. 2006. Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L. Chemosphere. 62:803-809.
- Mahimairaja S, Bolan NS, Adriano DC, Robinson B. 2005. Arsenic contamination and its risk management in complex environmental settings. Advances in Agronomy. 86:1-82.
- Pita AB, Gonçalves EC, Azevedo AA. 2015. Morpho-anatomical and growth alterations induced by arsenic in Cajanus cajan (L.) DC (Fabaceae). Environmental Science and Pollution Research. 22:11265-11274.
- Ronzan M, Piacentini D, Fattotini L, Eiche E, Riemann M, Altamura MM, Falasca G. 2018. Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environmental and Experimental Botany. 151:64-75.
- Singh HP, Batish DR, Kohli RK, Arora K. 2007. Arsenic-induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regulation. 53:65-73.