Authors:
- Fabiano Silvestrin, Principal Advisor, Global Market Development Agriculture
- Eduardo Saldanha, Agriculture Development Specialist, Latin America
The need for boron (B) as a plant nutrient in crop production, was first demonstrated in by Katherine Warington in 1923. We have 100 years of research and field trials showing the important role boron has in agriculture.
Soil plays a major role in determining the availability of B in plants:
- Texture: Well-drained, sandy soils in high rainfall regions are most likely to be B deficient because of their greater leaching potential.
- Organic matter content: Most of available B in soils is found in organic matter. Organic matter forms a complex with B to remove it from the soil solution when levels are high due to boron fertilization.
- Moisture retention: During periods of drought, the topsoil dries out so plant roots are unable to feed in the uppermost soil layer where most of the available B occurs.
- pH: B availability to plants generally decreases with increasing soil pH, especially above 6.5. However, acid soils (pH less than 5.0) also tend to be low in available B.
Summary of B adsorption in the soil:
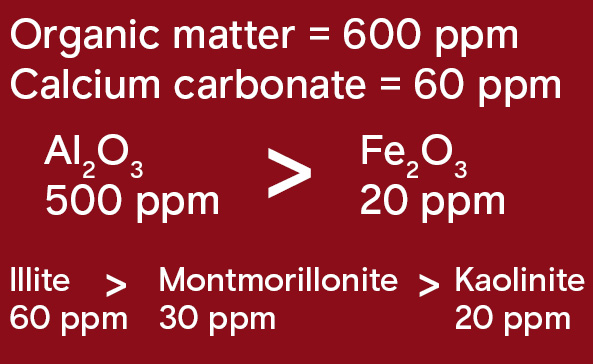
Modified from Yamada 2018.
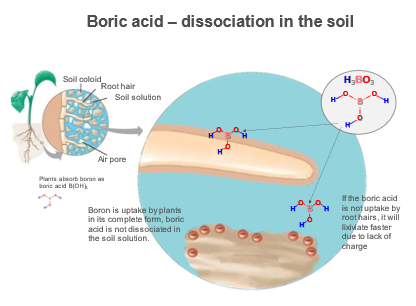
Boron is taken up by plant roots from the soil solution as uncharged boric acid (Marschner, 1995). Boric acid is a small molecule and is the only element which isn't absorbed from soil by plants as an ion. In the soil, boric acid (H3BO3) is transported to plant roots via mass flow—without water there is no movement of H3BO3 in the soil.
Once you apply borates to the soil, they will undergo chemical processes until they are transformed into H3BO3. Regardless of the type of boron applied to the soil—ulexite, colemanite, or borax pentahydrate—plant roots will always uptake boron in the form of H3BO3 by mass flow. The H3BO3 is then absorbed and translocated via xylem for the whole plant.
The direct application of boric acid to the soil has a greater potential for leaching into the soil because this element is in the form of H3BO3 and has no charge.
You may think that applying boric acid directly to the soil would be the best way to fix boron deficiency. But boric acid actually has a greater potential to cause plant toxicity compared to other B sources. Because boric acid is readily available to be taken up by plant roots, the plant will absorb it too quickly to be of use—causing the toxicity.
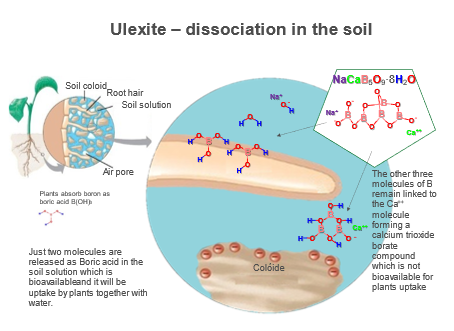
Source: U.S. Borax, 2018
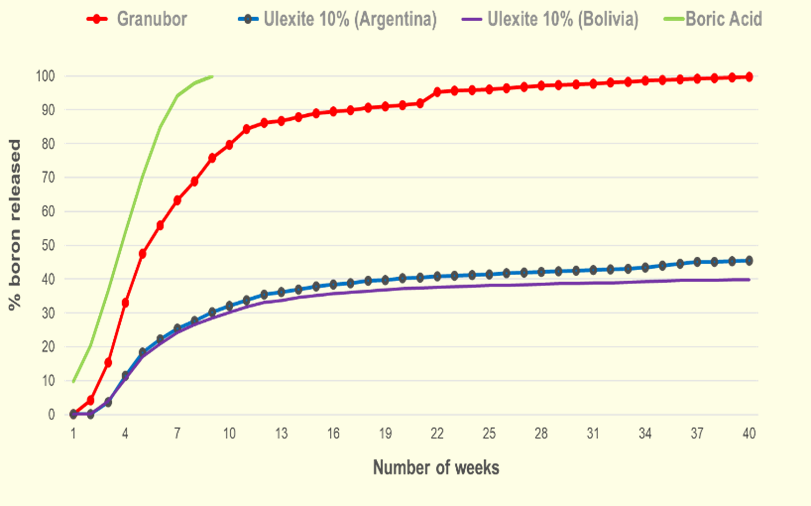
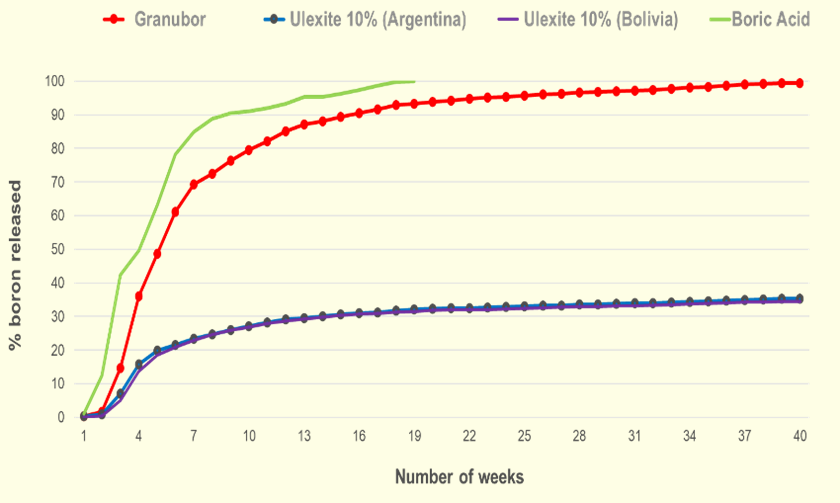
When ulexite (calcium-sodium borate) is applied to the soil, only 35 - 46% of the B present in this mineral will be released within 40 weeks, due to the strong bond between B and calcium (Ca).
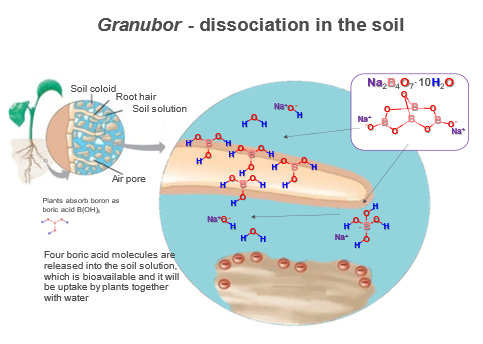
Source: U.S. Borax, 2018
When Granubor®—a sodium tetraborate pentahydrate product from U.S. Borax—is applied to the soil, almost 100% of B present in this fertilizer will be gradually released in the soil within 40 weeks. This happens due to the weak link between B and sodium (Na), giving enough time for crops uptake the released B.
Source: U.S. Borax, 2018
Study conducted over 40 weeks in sandy soil (pH 4.7) by Fundação ABC, Brazil
Study conducted over 40 weeks clay soil (pH 4.2) by Fundação ABC, Brazil
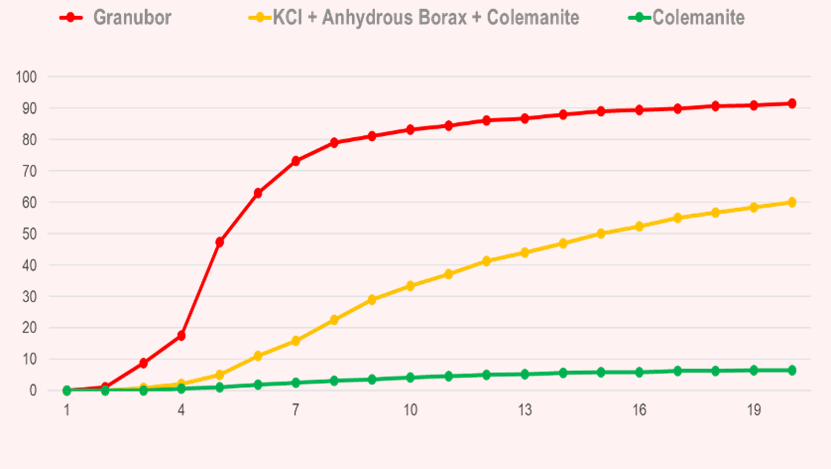
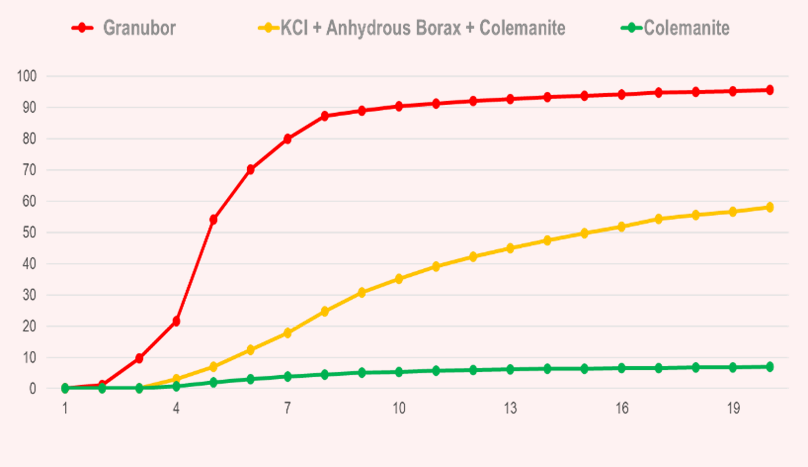
- When colemanite (calcium borate) is applied to the soil, less than 10% of the B present in this mineral will be released within 20 weeks, due to the strong bond between B and Ca.
- When compacted KCl (potassium chloride) + B (anhydrous borax + colemanite) is applied to the soil, approximately 60% of the B present in this fertilizer will be released within 20 weeks, due to the low solubility of colemanite and high solubility of anhydrous borax.
Study conducted over 20 weeks in sandy soil, and acidity corrected with ag lime (pH 5.7) by Fundação ABC, Brazil
Study conducted over 20 weeks in sandy soil and naturally acidic soil, without ag lime (pH 4.8) by Fundação ABC, Brazil
Summarized production process for boric acid
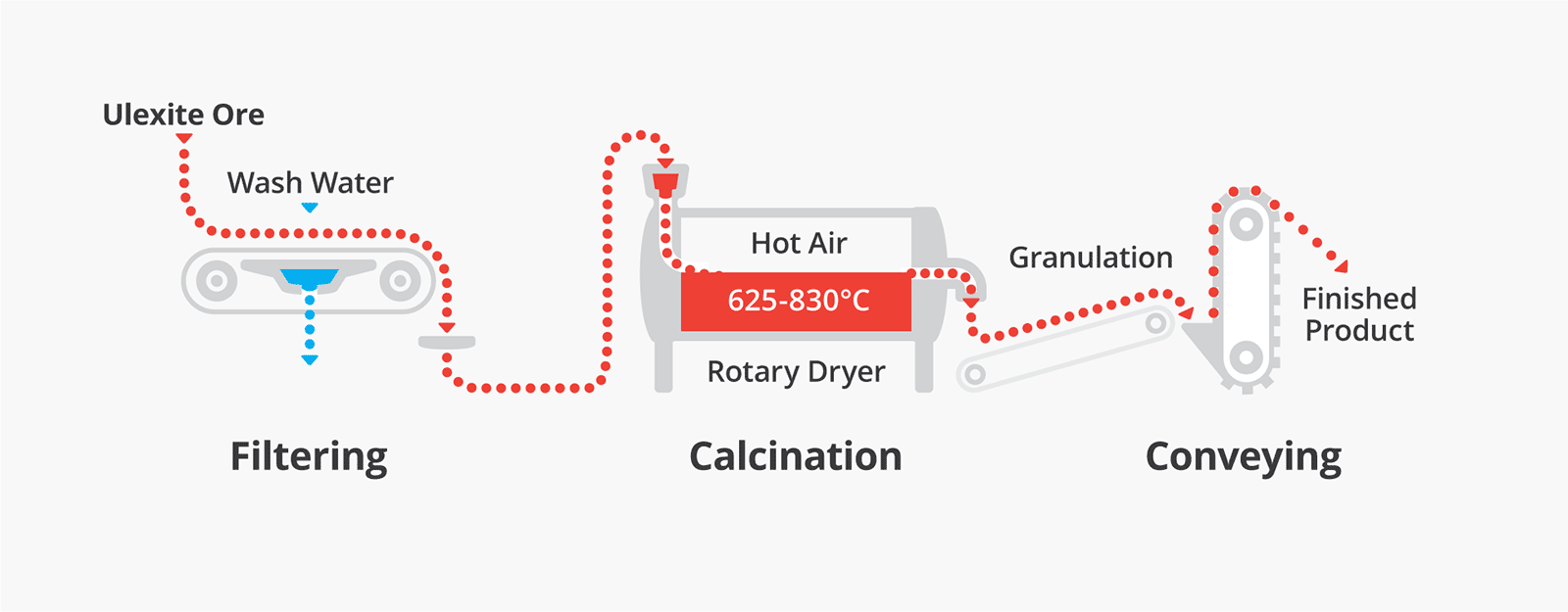
Production process for calcined ulexite
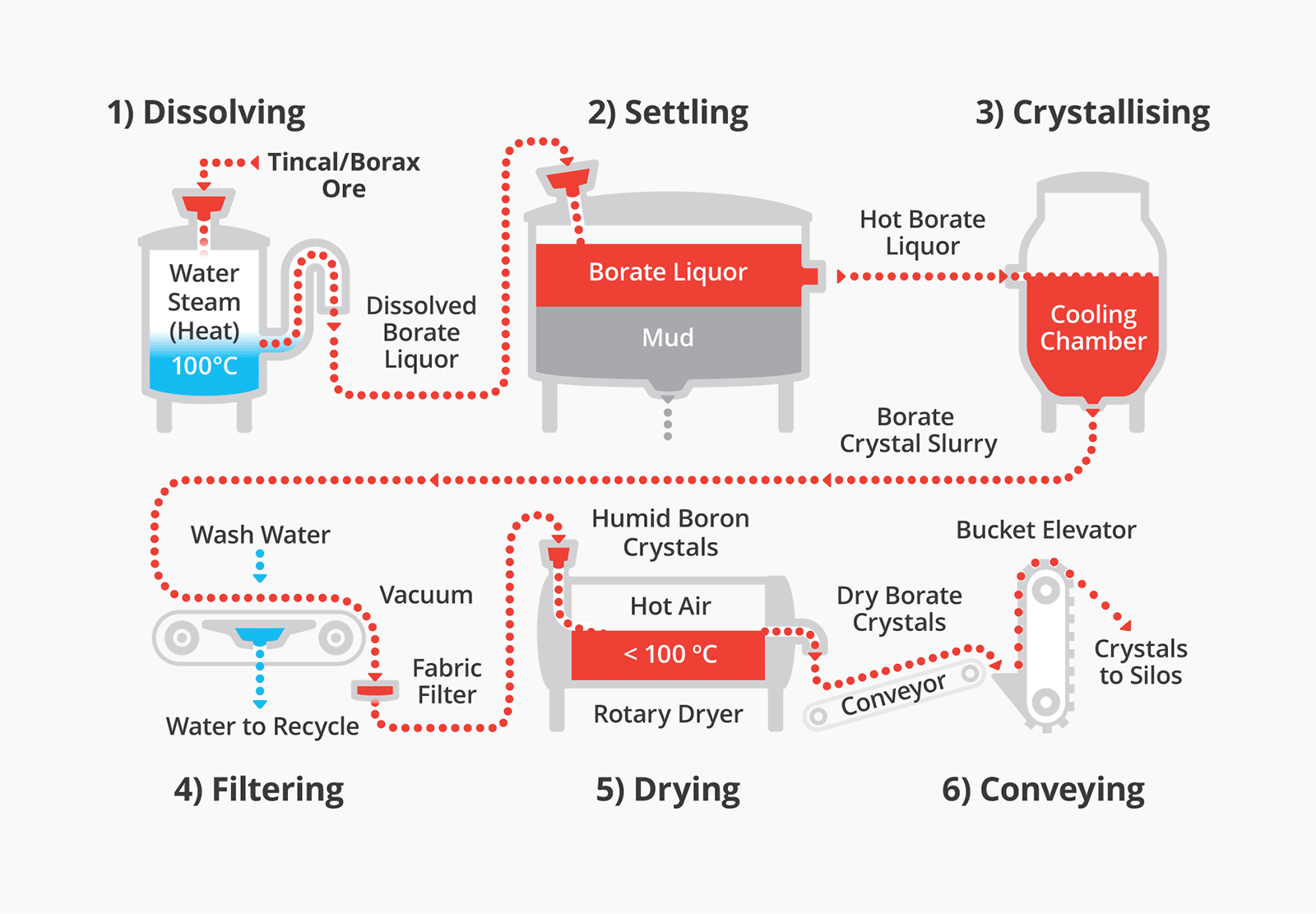
Production process for Granubor
Relationship of B and root system and the need for continuous supply
Crop productivity depends on different factors, including nutrient uptake, so attention should be paid to maintaining optimal levels of B in the soil solution.
There is an important relationship between B uptake and root architecture. Thin roots (lateral roots) contribute to nutrient absorption because they transport more than thick roots. Recent research has suggested that the primary effects of B-deficient plants result in rapid reduction of thin roots. The most immediate consequence is the atrophy and reduction of the volume of soil exploited by the roots, which translates into less absorption of water and other nutrients.
The accelerated degeneration of the root tissues, resulting from the complete inhibition of the root meristems, is quickly noticed at the growing points with the death of the apical buds, which effectively can negatively impact the entire metabolism, growth, and plant development. A continuous supply of boron is necessary for the maintenance of meristematic activity. Sources of B originating from refined borates, such as Granubor, can ensure the total and continuous supply of this element to the soil solution, keeping B levels at adequate levels to supply the nutritional demand of crops in this nutrient. The results obtained by Wojcik et al. 2008, studying forms of B application in apple trees, show that there was a significant effect of B supply in fine roots (Table 1), corroborating other research.
Table 1: Increase in root dry mass in apple plants
| Treatment |
B in the soil |
Root dry mass (g/plant) |
| |
mg/kg |
Thin roots |
Thick roots |
Total |
| Soil application |
0.56 a |
121 a |
35 a |
156 a |
| Control |
0.31 b |
<88 b |
31 a |
110 b |
Source: Wojcik et al. 2008
Recommendations for the use of boron
Interpretation of soil analysis
In the table below from the Agronomic Institute of Campinas (IAC), there are the indications of the B availability classes in the soil. These classes are listed in the publication Boletim 100, considered the most current publication in Brazil. The classes are segmented for annual and perennial crops and are indicated below:
Availability classes for B in soil: Annual crops
| Availability class |
B (hot water)
mg/dm3 |
| Low |
<0.2 |
| Medium |
0.2-0.6 |
| High |
>0.6 |
Availability classes for B in soil: Perennial crops
| Availability class |
B (hot water)
mg/dm3 |
| Low |
<0.6 |
| Medium |
0.6-1.0 |
| High |
>1.0 |
Time of application and method of location
Boron applications should be carried out after identifying where its needed, using tools to assess soil fertility, and the nutritional status of crops. These techniques will help define agronomic recommendation criteria. Boron applications can be carried out at planting and in coverage, depending on the crop. The timing of applications must be adjusted in such a way that they meet the B needs and the vegetative and reproductive phases—the latter being the most demanding for crops. Field applications can be mechanized or with manual applications.
Application frequency
Special attention should be given to boron applications in soils with a sandy texture (less than 15% clay), as the retention capacity of this nutrient is lower, which may result in a reduction in the soluble levels of the element in the soil solution. In these soils, the doses must be split to maximize the efficiency of the fertilization and use by the plants. The frequency of applications will also depend on the crop fertilization program. Soil applications can be complemented with foliar applications, especially in periods of strong demand for B, such as flowering and fruit formation.
Dose
The indicated doses vary according to the available levels of B in the soil and the demand of the crops. Bulletin 100 illustrates an indication for different crops. Here are just a few crops:
| Crop |
Application |
Doses of B (kg/ha)** |
| Citrus |
Via soil and foliar
(complementary) |
2.0-3.0 |
| Coffee |
Via soil |
1.0-4.0 |
| Corn |
Via soil and foliar
(complementary) |
2.0 |
| Cotton |
Via soil |
1.0-2.5 |
| Eucalyptus |
Via soil |
3.2-8.5 |
| Soybean |
Via soil |
1.0 |
| Sunflower |
Via soil |
1.0-2.0 |
*Productive phase
**Depending on the availability class of B
Sources
The choice of source is very important in borate nutrition. Fertilizers that guarantee a total and gradual supply of B, such as Granubor, are recommended as they ensure a continuous supply of nutrients throughout the cycle. Foliar complements can be performed with Solubor® and Solubor Flow from U.S. Borax. Here are some technical properties:
| Source |
Formulations |
B content (%) |
Solubility (g/l) |
Use |
| Granubor |
Na2B4O7·5H2O |
15 |
26.5 |
Soil application |
| Solubor |
Na2B8O13·4H2O |
20.5 |
97 |
Foliar and fertigation |
Resources
References
- Cantarella H, Quaggio JA, Mattos Jr. D, et al. 2022. Boletim 100: Recomendações de adubação e calagem para o estado de São Paulo. Instituto Agronômico de Campinas – IAC:490.
- Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Ro. 2022. Boron in plant biology. Plant Biology: 205–223.
- Marschner H. 1995. Functions of mineral nutrients: micronutrients. Marschner H, editor. Mineral Nutrition of Higher Plants (2nd ed). London: Academic Press. p. 313–404.
- Wojcik P, Wojcok M, Klamkowski K. 2008. Responde of apple trees to boron fertilization under conditions of low soil boron availability. Scientia Horticulturae.116:58-64.